
Right for cattle. Right by you.
Right for cattle. Right by you.
The beef industry is confronted by challenges new and old. Elanco is committed to enabling its success by providing resources to help manage health, economic performance and operational sustainability.
Our products are supported by Elanco representatives and technical consultants to help operations achieve their herd health, economic productivity and operational sustainability goals.
Solutions tailored to your needs
Whether you’re a cow-calf operator, stocker operator or managing a feedyard, Elanco provides solutions for healthy, productive cattle that sustain the success of your business and our industry. We’re committed to providing resources to help manage key diseases, minimize parasite impact, optimize productivity and improve environmental stewardship.
 Cow-Calf
Cow-CalfStart calves off for a lifetime of productivity.
 Stocker
StockerElanco offers tools for health and weight gain.
 Feedlot
FeedlotMaximize endpoint outcomes with Elanco resources.
Elanco Beef Products Portfolio
Elanco provides trusted solutions that yield healthy cattle, economic performance and sustainable operations.


Baytril® 100 (enrofloxacin)
Baytril 100 is a proven tool for BRD treatment and control in beef and non-lactating dairy.

BRD Portfolio
Elanco offers a comprehensive portfolio of proven solutions to prevent, treat and control BRD.


Component®
Adaptable range of seven cattle implants, including five that feature the Tylan pellet.


Cydectin® (moxidectin) Pour-On
The only milbemycin cattle dewormer on the market that controls 33 parasites and stages.

Experior™ (lubabegron)
The first FDA approved product labeled to reduce ammonia gas emissions from cattle.

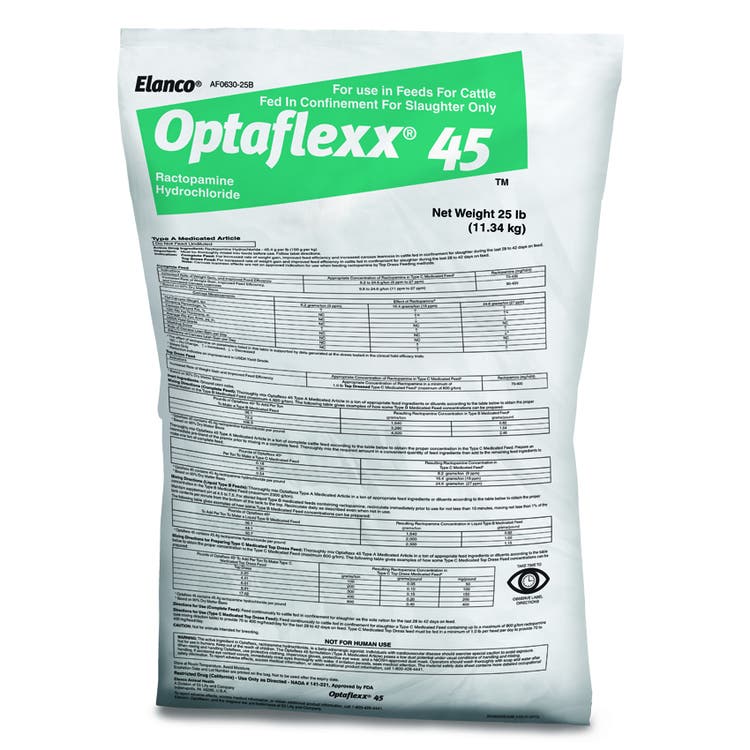
Optaflexx®
Optaflexx increases live weight gain, hot carcass weight and improves feed efficiency.

Rumensin®
Improve feed efficiency, increase gain and prevent and control coccidiosis with Rumensin.

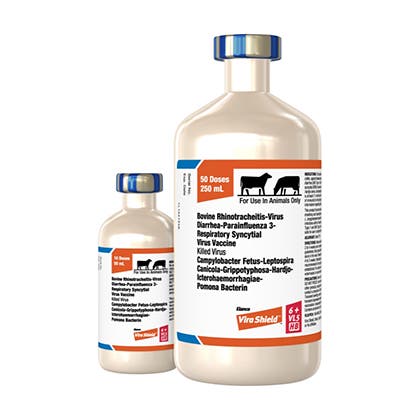
Vira Shield®
A safe and powerful line of inactivated vaccines that provide protecion against up to 13 respiratory and reproductive diseases.

Cattle Fly Control
Elanco offers comprehensive fly control solutions, backed by parasiticide application and expertise.
How to Contact Us
To find out more about our products and services, please contact one of our knowledgeable representatives today.
Keep Cydectin out of reach of children.
FOR ALL PRODUCTS: The label contains complete use information, including cautions and warnings. Always read, understand and follow the label, and use directions.
Baytril 100
Cautions:
Not for human use. Keep out of reach of children. For subcutaneous use in beef cattle and non-lactating dairy cattle.
Federal (U.S.A.) law restricts this drug to use by or on the order of a licensed veterinarian. Federal (U.S.A.) law prohibits the extra-label use of this drug in food-producing animals.
Indications:
Cattle – Single-Dose Therapy: Baytril 100 is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis in beef and non-lactating dairy cattle; and for the control of BRD in beef and non-lactating dairy cattle at high risk of developing BRD associated with M. haemolytica, P. multocida, H. somni and M. bovis.
Cattle – Multiple-Day Therapy: Baytril 100 is indicated for the treatment of BRD associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni in beef and non-lactating dairy cattle.
Directions for use:
Single-Dose Therapy (BRD Treatment): Administer, by subcutaneous injection, a single dose of 7.5-12.5 mg/kg of body weight (3.4-5.7 mL/100 lbs.).
Multiple-Day Therapy (BRD Treatment): Administer daily, a subcutaneous dose of 2.5-5 mg/kg of body weight (1.1-2.3 mL/100 lbs.).
Treatment should be repeated at 24-hour intervals for three days. Additional treatments may be given on days 4 and 5 to animals that have shown clinical improvement but not total recovery.
Baytril 100 provides flexible subcutaneous injection dosages and durations of therapy.
Do not use in female dairy cattle 20 months of age or older or in calves to be processed for veal.
Talk with your veterinarian to learn more.
Component with Tylan
Directions for use:
Administer one dose in the ear subcutaneously according to label directions.
Experior
Cautions:
Not approved for use in breeding animals because safety and effectiveness have not been evaluated in these animals. Do not allow horses or other equines access to feed containing Experior. A decrease in dry matter intake may be noticed in some animals.
Indications:
For the reduction of ammonia gas emissions per pound of live weight and hot carcass weight in beef steers and heifers fed in confinement for slaughter during the last 14 to 91 days on feed.
Directions for use:
Feed. 1.25 to 4.54 g/ton (1.39 to 5 ppm) of complete feed (90% dry matter basis) to provide 13-90 mg lubabegron/head/day continuously to beef steers and heifers fed in confinement for slaughter as sole ration during the Last 14 to 91 days on feed.
Ammonia gas emissions were measured for individual animals or small groups of animals held in environmentally controlled facilities. Based on existing information, reliable predictions of the reduction of ammonia gas emissions cannot be made on a herd, farm, or larger scale. Increased rate of weight gain, improved feed efficiency, and increased carcass leanness have not been demonstrated with this product.
Optaflexx
Caution:
Not for animals intended for breeding.
Directions for use:
Complete feed
For increased rate of weight gain and improved feed efficiency in cattle fed in confinement for slaughter: Feed 8.2 to 24.6 g/ton of ractopamine hydrochloride (90% DM basis) continuously in a complete feed to provide 70 to 430 mg/hd/d for the last 28 to 42 days on feed.
For increased rate of weight gain, improved feed efficiency and increased carcass leanness in cattle fed in confinement for slaughter: Feed 9.8 to 24.6 g/ton of ractopamine hydrochloride (90% DM basis) continuously in a complete feed to provide 90 to 430 mg/hd/d for the last 28 to 42 days on feed.
Top dress
For increased rate of weight gain and improved feed efficiency in cattle fed in confinement for slaughter: Feed 70 to 400 mg/hd/d of ractopamine hydrochloride (90% DM basis) continuously in a minimum of 1.0 lb/hd/d top dress Type C medicated feed (maximum 800 g/ton ractopamine hydrochloride) during the last 28 to 42 days on feed.
Carcass leanness effects are not an approved indication for use when feeding ractopamine by top dress feeding methods.
Rumensin
Cautions:
Consumption by unapproved species or feeding undiluted may be toxic or fatal. Do not feed to veal calves.
Directions for use:
Growing beef steers and heifers fed in confinement for slaughter:
For improved feed efficiency: Feed 5 to 40 g/ton of monensin (90% DM basis) continuously in a complete feed to provide 50 to 480 mg/hd/day.
For the prevention and control of coccidiosis due to Eimeria bovis and Eimeria zuernii: Feed 10 to 40 g/ton (90% DM basis) to provide 0.14 to 0.42 mg/lb. of body weight/d, depending upon severity of challenge, up to a maximum of 480 mg/hd/day.
Growing beef steers and heifers on pasture (stocker, feeder and slaughter) or in a dry lot, and replacement beef and dairy heifers:
For increased rate of weight gain: Feed 50 to 200 mg/hd/day in at least 1.0 lb. of Type C Medicated Feed. Or, after the fifth day, feed 400 mg/hd/day every other day in 2.0 lbs. of Type C Medicated Feed. The Type C Medicated Feed must contain 15 to 400 g/ton of monensin (90% DM basis). Do not self-feed.
For the prevention and control of coccidiosis due to Eimeria bovis and Eimeria zuernii: Feed at a rate to provide 0.14 to 0.42 mg/lb. of body weight/day, depending upon the severity of challenge, up to a maximum of 200 mg/hd/day. The Type C Medicated Feed must contain 15 to 400 g/ton of monensin (90% DM basis).
Type C free-choice medicated feeds: All Type C free-choice medicated feeds containing Rumensin must be manufactured according to an FDA-approved formula/specification. When using a formula/specification published in the Code of Federal Regulations (CFR), a Medicated Feed Mill license is not required. Use of Rumensin in a proprietary formula/specification not published in the CFR requires prior FDA approval and a Medicated Feed Mill License.
Beef cows:
For improved feed efficiency when receiving supplemental feed: Feed continuously at a rate of 50 to 200 mg/hd/day. Cows on pasture or in dry lot must receive a minimum of 1.0 lb. of Type C medicated feed/hd/d. Do not self-feed.
For the prevention and control of coccidiosis due to Eimeria bovis and Eimeria zuernii: Feed at a rate of 0.14 to 0.42 mg/lb. of body weight/d, depending upon severity of challenge, up to a maximum of 200 mg/hd/day.
For calves (excluding veal calves):
For the prevention and control of coccidiosis due to Eimeria bovis and Eimeria zuernii: Feed at a rate of 0.14 to 1.00 mg/lb. of body weight/day, depending upon the severity of challenge, up to a maximum of 200 mg of monensin/hd/day. The monensin concentration in Type C medicated feed must be between 10 and 200 g/ton.
Component, Cydectin, Experior, Nuplura, Optaflexx, Rumensin, Tylan, Vira Shield, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
Baytril is sold by Elanco or its affiliates and is not a Bayer product. The Baytril trademark is owned by Bayer and used under license.
Click to download Baytril 100 Product Label.